Published online Oct 26, 2016. doi: 10.4330/wjc.v8.i10.606
Peer-review started: May 9, 2016
First decision: June 13, 2016
Revised: July 23, 2016
Accepted: August 30, 2016
Article in press: August 31, 2016
Published online: October 26, 2016
To investigate the accuracy of a rotational C-arm CT-based 3D heart model to predict an optimal C-arm configuration during transcatheter aortic valve replacement (TAVR).
Rotational C-arm CT (RCT) under rapid ventricular pacing was performed in 57 consecutive patients with severe aortic stenosis as part of the pre-procedural cardiac catheterization. With prototype software each RCT data set was segmented using a 3D heart model. From that the line of perpendicularity curve was obtained that generates a perpendicular view of the aortic annulus according to the right-cusp rule. To evaluate the accuracy of a model-based overlay we compared model- and expert-derived aortic root diameters.
For all 57 patients in the RCT cohort diameter measurements were obtained from two independent operators and were compared to the model-based measurements. The inter-observer variability was measured to be in the range of 0°-12.96° of angular C-arm displacement for two independent operators. The model-to-operator agreement was 0°-13.82°. The model-based and expert measurements of aortic root diameters evaluated at the aortic annulus (r = 0.79, P < 0.01), the aortic sinus (r = 0.93, P < 0.01) and the sino-tubular junction (r = 0.92, P < 0.01) correlated on a high level and the Bland-Altman analysis showed good agreement. The interobserver measurements did not show a significant bias.
Automatic segmentation of the aortic root using an anatomical model can accurately predict an optimal C-arm configuration, potentially simplifying current clinical workflows before and during TAVR.
Core tip: We were able to demonstrate the accuracy of a rotational C-arm CT (RCT) based 3D heart model to predict an optimal C-arm configuration and to provide anatomical context information during transcatheter aortic valve replacement (TAVR). Established and upcoming complex cardiac interventions require detailed anatomical information for procedure planning and intra-procedural guidance. According to our experience, RCT can be smoothly integrated into the clinical workflow, providing three-dimensional information of the relevant anatomical structures in the catheterization lab prior and as part of the TAVR intervention.
- Citation: Veulemans V, Mollus S, Saalbach A, Pietsch M, Hellhammer K, Zeus T, Westenfeld R, Weese J, Kelm M, Balzer J. Optimal C-arm angulation during transcatheter aortic valve replacement: Accuracy of a rotational C-arm computed tomography based three dimensional heart model. World J Cardiol 2016; 8(10): 606-614
- URL: https://www.wjgnet.com/1949-8462/full/v8/i10/606.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i10.606
Transcatheter aortic valve replacement (TAVR) is an established treatment option for patients ineligible for surgery that suffer from severe aortic stenosis[1,2]. Optimal positioning of the prosthetic valve during the intervention in the catheter laboratory is crucial for procedural success. Malpositioning may lead to valve embolization, coronary ostial obstruction, perivalvular regurgitation, or conduction disturbances[3]. Optimal and safe device deployment is best accomplished by generating a specific fluoroscopic view perpendicular to the annulus plane, also known as the line of perpendicularity (LP)[4]. To achieve this specific fluoroscopic view during the TAVR procedure, several angiograms in different angulations of the C-arm are necessary, causing a considerable amount of nephrotoxic contrast agent and radiation for the patient and the operator[5]. Therefore, an accurate definition of the aortic annulus and the LP is desirable before the procedure is performed. Today, MSCT is the preferred modality for TAVR planning and intervention guidance, providing information about anatomic conditions as well as the opportunity to reformat the reconstruction in any 3D orientation[6,7].
Different imaging techniques have been established to define the LP optimal fluoroscopic view during the preprocedural screening of patients. For angiography and MSCT[7-10] different software solutions for optimal view planning and their clinical benefits have been proposed. Automated view planning along the LP has shown to improve the quality of implantation, may speed up workflow and may reduce the need for low-dose aortograms[5]. Rotational C-arm computed tomography (RCT)-based view planning has proven to be of equal quality as MSCT-based techniques[10-12]. But current studies purely rely on non-quantitative evaluations and systematic validation of software-based methods is lacking.
In this study we therefore sought to (1) evaluate the accuracy of a RCT based 3D heart model for segmentation of the aortic root to predict an optimal C-arm configuration that generates a perpendicular view of the aortic annulus during TAVR and (2) investigate whether the accuracy of a RCT-specific model is suitable for intervention guidance, comparing the dimensions of an automatically derived overlay with manual reference measurements.
Retrospectively, 57 consecutive patients (30 male, mean age 80.9 years) with symptomatic severe aortic stenosis that underwent cardiac catheterization with RCT prior to planned TAVR or surgical aortic valve replacement (SAVR) procedure have been selected. Patients with insufficient RCT image quality, e.g., due to incomplete RVP (n = 2), delayed contrast timing (n = 2), massive artefacts by ICD (n = 2) were excluded beforehand. All patients gave written consent and the study was approved by the local ethics committee (Study No. 4080, international registration NCT01805739).
RCT was performed as part of the pre-implant diagnostic coronary angiography study[13]. The C-arm of the Cathlab system (Allura FD 20, 30 cm flat panel detector, XperCT option, Philips Healthcare, Andover, MA, United States) was rotated over an angular range of 210° with a sweep duration of 5.2 s and a frame-rate of 60 frames/s around the patient. To mitigate motion the acquisition was conducted during inspiratory breath hold and under RVP. Contrast medium (Accupaque 350, Bracco Imaging, Konstanz, Germany) was diluted 1:1 with saline to a total volume of 0.8 mL/kg patient’s weight (50-80 mL) and administered with a flow rate of 14 mL/s. The contrast agent was injected via a pigtail catheter either supravalvular into the ascending aorta aortic root or subvalvular into the left ventricular cavity. The rotational sweep data was reconstructed with standard product settings to a volume of size 256 × 256 × 198 with an isotropic resolution of 0.98 mm³. Since the RCT acquisitions were performed during RVP the exact cardiac phase cannot be specified.
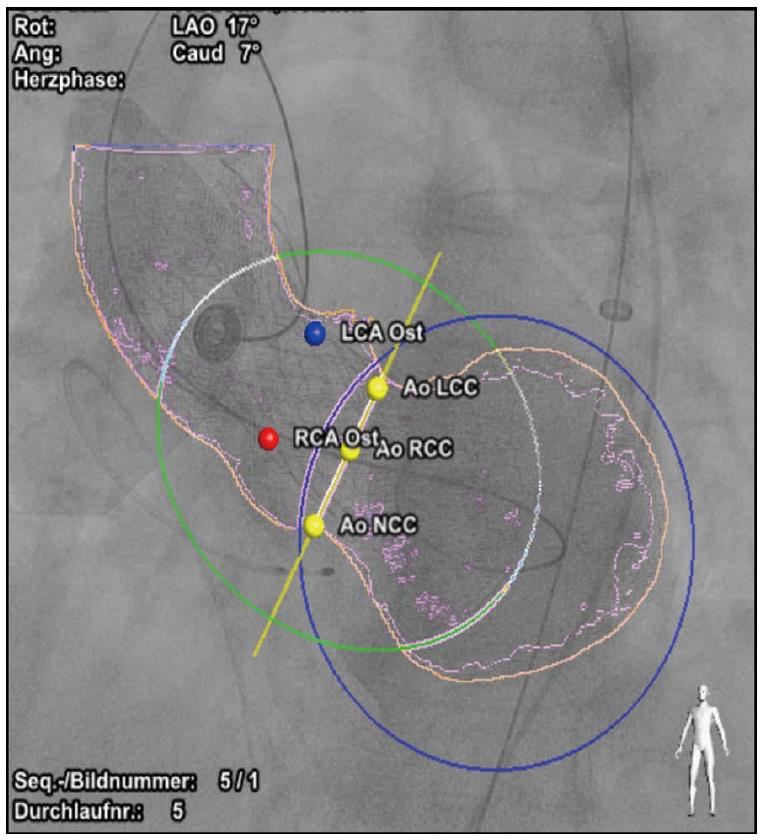
To assess the operator-variability and the accuracy of software-based optimal C-arm configurations, reference views were defined by a medical expert. Three-dimensional reconstructions of the RCT were visualized as multi-planar reformats with proprietary prototype software. Two blinded operators, experienced in the analysis of cardiac cross sectional imaging, used standard volume interaction techniques to manually define a view perpendicular to the aortic valve plane with respect to a reference viewport. From this optimal view, a LP curve was automatically derived using the mathematical definitions below and the result was presented to the user. Based on the LP curve and a volume rendering of the original RCT data set, the operators defined an optimal C-arm configuration in terms of rotation and angulation following the right-cusp rule[14].
Furthermore the RCT data sets were studied with vendor-independent image processing software (Osirix MD Ver. 4.0, pixmeo, Geneva, Switzerland). Two independent, blinded observers performed aortic root diameter measurements in multiplanar reformatted RCT data sets at the level of the aortic annulus, the aortic sinus and the sino-tubular junction (STJ). These are supposed to be representative for the shape and dimensions of the aortic root anatomy to be overlaid to the fluoroscopic data stream for intervention guidance[13].
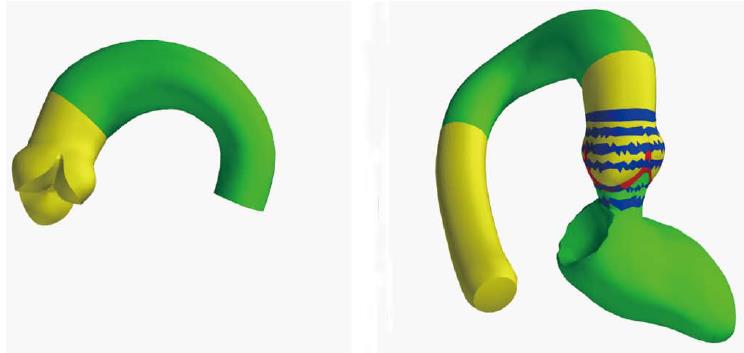
For automatic view planning and intervention guidance in RCT, a model-based segmentation technique was employed[15]. Unlike other segmentation techniques, model-based segmentation integrates information about the typical shape of the target anatomy, its variability and appearance in the adaptation process, and has been successfully employed in a broad range of medical image processing applications[16-18]. To tailor the shape model to the image characteristics of RCT the model was trained on the 57 patients of the RCT cohort whereby the validation was set-up in a leave-N-out manner so that training and test set never coincided. The shape model covers the 3D outline of the aortic valve, the supravalvular part of the aorta, the aortic arch, a list of anatomical landmarks and rings encoded on the mesh model that enable geometrical measurements relevant for the TAVR application (Figure 1). For clinical validation each RCT data set was segmented with our prototype software using the 3D heart model. The aorta, the aortic valve and the left ventricle (if visible in the RCT data set) as well as the nadir landmarks of the three aortic valve cusps were extracted. From that, the LP curve was obtained and an optimal view that aligns the nadir landmarks according to the right-cusp rule was computed.
To compute the accuracy of the model-based overlay for intervention guidance we assume that the shape and the dimension of the aortic root can be roughly represented by a set of diameter measurements. These diameter measurements use the rings encoded on the segmentation model and are defined in accordance with the recommendations of the manufacturers of the TAVR devices. For the diameter of the annulus a circular cross-section model is fit to the segmentation result. The measurement of the bulbus width and the diameter of the STJ rely on an elliptical cross-section model.
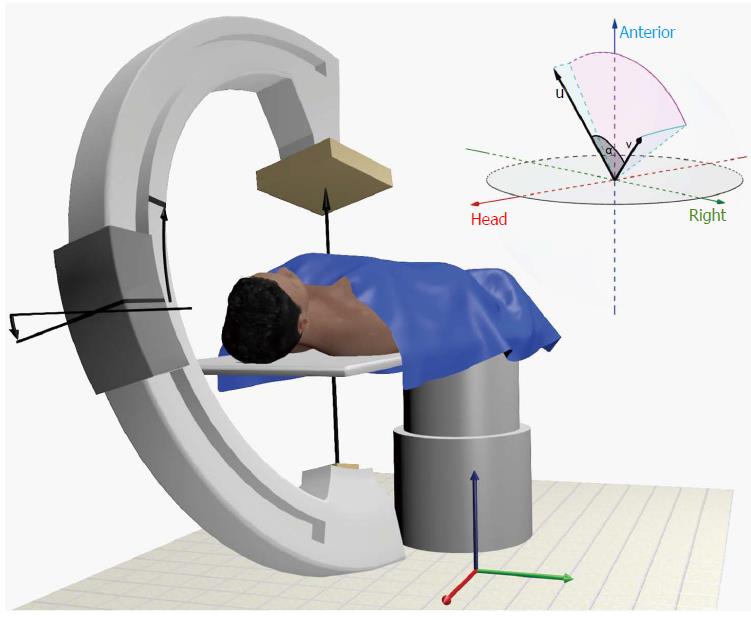
The computation of the LP curve and the error computations require several geometrical definitions. Prerequisite is a Cartesian coordinate system which is defined in analogy to the work of Wollschläger et al[19]. The origin of the coordinate system coincides with the isocenter of the C-arm system. As in Figure 2 indicated, the C-arm can be angulated along the x-axis in cranial and caudal direction of the supine patient and is able to rotate along the y-axis in LAO and RAO direction. The z-axis is defined in dorsal-ventral patient orientation. One pair of rotation and angulation denoted as (,θ) can be represented by a vector in the C-arm coordinate system v_(,θ) = (x, y, z) where x = sin (θ), y = sin ()∙cos (θ), z = cos ()∙sin (θ). Each combination of rotation and angulation spans a virtual half-sphere around the patient. In this half-sphere the LP curve is represented as trace of C-arm rotation and angulation combinations. Each respective view along this trace is orthogonal to the axial plane of the patient’s aortic valve which can be defined by the unit vector v_AV = (x_AV, y_AV, z_AV). To compute the LP curve we seek for a given C-arm rotation θ the C-arm angulations so that the vectors v_(,θ) and v_AV are perpendicular. This can be expressed as inner product of two vectors which is set to zero v_(,θ)∙v_AV = 0.
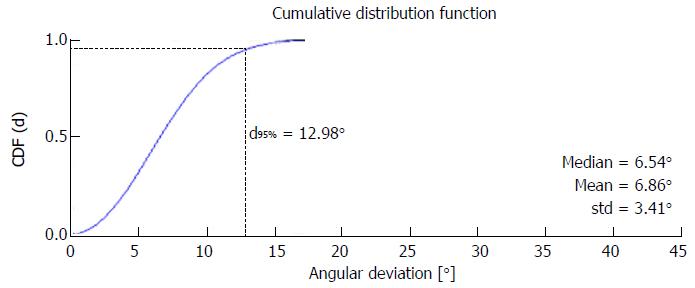
To evaluate the inter-observer variability and the agreement between model-based and expert-defined optimal views we compute the angular deviation (AD) between the respective position vectors v_model and v_expert in the spherical C-arm coordinate system which can be expressed as α = acos (v_model ¡Á v_expert). In analogy to the work of the Tzikas-group[8], we compute the mean absolute difference and the standard deviation of the angular deviations between the position vectors given by the operators and the RCT model for all patients. However, this form of statistical analysis is error-prone, since it assumes the normal distribution of the random samples. But the angulation and rotation parameters are dependent on each other and further numerical restrictions (such as pole of acos-function near the optimal vector configuration) have to be considered. Thus, we propose to use a more advanced method of error calculation well-known from other research fields[20]. Therefore we apply Monte-Carlo methods to compute the cumulative distribution function of the angular deviations and use the value at 95% confidence level for the error calculation.
The Bland-Altman method was used for the assessment of the bias and standard deviations between model-based and expert-based aortic root measurements in RCT at 95% level of agreement (LoA). In addition, the Pearson correlation coefficient r was computed and t statistics were used to test the hypothesis of no correlation considering a significance level of p < 0.01. All statistical calculations were performed using Matlab Statistics Toolbox™ (MathWorks, Inc, Natick, Massachusetts, United States).
For optimal view planning 57 patients with RCT were evaluated. To assess the inter-observer variability the angular deviations between two expert-defined views in the RCT patient cohort were computed. Assuming normal distribution of the angular deviations, the inter-observer variability was measured to be 7.05°± 3.06° and thus, in the same range as reported in the work of Tzikas et al[8]. Using Monte Carlo methods an interobserver variability between 0° and 12.96° was obtained (compare Table 1 and Figure 3). Furthermore we compared the view planning results of our prototype software with the expert definitions. The model-operator agreement jointly computed for both operators was 6.84°± 3.78° assuming normal distribution and 0°-13.82° for the Monte Carlo method and thus, on a similar level as the inter-observer variability. A sample LP curve and the respective optimal views of two operators and the prototype software are given in Figure 4.
| n = 57 | Average AD (ND) | Average AD (MC) |
| Operator 1 vs operator 2 | 7.05°± 3.06° | 12.96° |
| RCT model vs both operators | 6.84°± 3.78° | 13.82° |
| RCT model vs operator 1 | 7.14°± 4.12° | 14.37° |
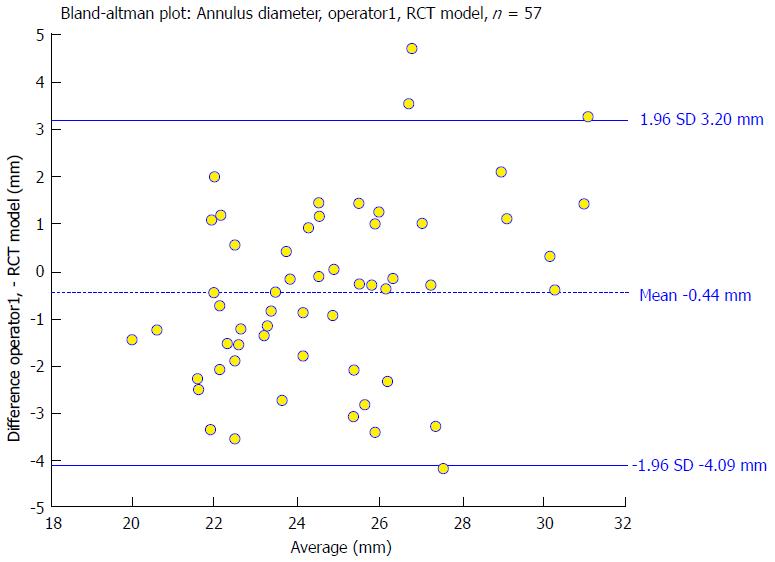
To evaluate the accuracy of RCT-based overlays to interventional data, the dimension of the aortic root at the level of the aortic annulus, the sinus and the STJ was studied. For all 57 patients in the RCT cohort diameter measurements were obtained from two independent operators and were compared to the model-based measurements. For the aortic annulus the Bland-Altman analysis showed no trend for under- or over-estimation comparing the model-based segmentation results with the expert measurements (mean difference for model vs operator 1: -0.44 mm, LoA: -4.09 mm to 3.2 mm). The correlation was significant (r = 0.79). A sample Bland-Altman plot is given in Figure 5. For the aortic sinus width and STJ diameter measurements the scatter and the limits of agreement were slightly smaller and the correlation levels higher as listed in Table 2. The Bland-Altman analysis for the aortic sinus diameter shows a good agreement between model-based and medical expert measurements with a bias of 1.05 mm using RCT and limits of agreement that range from -1.64 mm to 3.75 mm for operator 1. Correlations between expert and model-based measurements varied between 0.93 and 0.96. The results of the STJ diameter measurements show a slight bias of -1.53 mm and the limits of agreement were -4.21 mm to 1.15 mm for operator 1. Model-based and expert measurements correlated on a high level (operator 1: r = 0.92; operator 2: r = 0.93). The interobserver measurements did not show a significant bias. Scatter and correlation levels were for all studied parameters in the same range as the model-operator measurements.
| n = 57 | Annulus | Sinus | STJ | ||||||
| Bias | LoA | r | Bias | LoA | r | Bias | LoA | r | |
| Operator vs operator 1 | 0.32 | -3.17-3.81 | 0.81 | -0.45 | -3.61-2.71 | 0.91 | -0.59 | -3.29-2.10 | 0.92 |
| RCT model vs operator 1 | -0.44 | -4.09-3.20 | 0.79 | 1.05 | -1.64-3.75 | 0.93 | -1.53 | -4.21-1.15 | 0.92 |
| RCT model vs operator 2 | -0.76 | -3.75-2.23 | 0.81 | 1.51 | -0.61-3.62 | 0.96 | -0.94 | -3.41-1.53 | 0.93 |
Different imaging techniques have been established to define the optimal fluoroscopic view and to optimize valve deployment during TAVR. Standard to define a perpendicular view of the aortic valve is the repeated acquisition of aortographies from different projection angles. During recent years several software solutions for automatic view planning mainly on the basis of MSCT have been developed and have demonstrated high accuracy and many clinical benefits[5].
However, the collection of a MSCT data set for TAVR view planning involves extra logistics for the clinic and additional burden and hazards for the patient. Rotational C-arm CT has proven to be a useful imaging technique for many clinical applications[21] but is less established in the context of TAVR. The image quality of C-arm CT is generally limited by the acquisition quality and thus model-based view planning are dependent on accurate contrast agent bolus timing and on sufficient rapid pacing protocols. According to our clinical experience we believe that with more widespread use and maturity in future, rotational C-arm based imaging can play a more significant role in the TAVR workflow in combination with software-based view planning support.
In this study we evaluated the accuracy of automated view planning with RCT. We could show that our novel prototype software estimates optimal views on the basis of RCT data with good accuracy and that the interobserver variability and model-operator agreement are in the same range. Although different contrast agent injection protocols (aortic root injection vs left-ventricular injection) were part of the RCT validation cohort the model-based view planning in RCT has proven to be robust.
The current standard for intervention guidance during TAVR is plain fluoroscopy. In recent years software such as the HeartNavigator software (Philips Healthcare, Andover, MA, United States; compare Figure 6) that segments a three-dimensional MSCT data set to create a patient-specific model of the heart and overlays this to the interventional image stream has been developed. In this study we examined the accuracy of RCT-based overlays that are automatically generated from model-based segmentation. We found that our RCT-based techniques are able to accurately reflect the dimension of the aortic valve annulus and the aortic root. Bias and variations of model-based measurements vs the experts’ references were in the same range as the operator variability. Thus, RCT modeling can potentially provide accurate anatomical overlays to interventional data to support the TAVR intervention as current software solutions already do for MSCT.
In conclusion, established and upcoming complex cardiac interventions such as TAVR require detailed information regarding heart and vessel anatomy for procedure planning and intra-procedural guidance. According to our experience, rotational C-arm CT can be smoothly integrated into the clinical workflow, providing three-dimensional information of the relevant anatomical structures in the catheterization lab prior and as part of the TAVR intervention.
This study was based on retrospective data and reflects solely the experience at our center. The RCT data was acquired during TAVR/SAVR procedure planning several days in advance to the procedure. The data in this study were based on a relatively small sample size to show the clinical feasibility. Possible clinical benefits have to be investigated in prospective studies with more standardized protocols and a more powerful sample size. Future studies should prove feasibility and accuracy of RCT acquisition as initial step during TAVR procedure which may increase accuracy of view planning and intervention guidance further due to fewer patient position changes. In addition, RCT-based calcium visualization and quantification has to be studied.
Optimal positioning of the prosthetic valve is crucial for procedural success of Transcatheter aortic valve replacement (TAVR). Optimal and safe device deployment is best accomplished by generating a specific fluoroscopic view perpendicular to the annulus plane, also known as the line of perpendicularity (LP). To achieve this specific fluoroscopic view during the TAVR procedure, several angiograms in different angulations of the C-arm are necessary, causing a considerable amount of nephrotoxic contrast agent and radiation for the patient and the operator. Different imaging techniques have been established to define the LP optimal fluoroscopic view during the preprocedural screening of patients. Multi-slice computed tomography (MSCT) is the preferred modality and “gold-standard” for TAVR planning and intervention guidance. For angiography and MSCT different software solutions for optimal view planning and their clinical benefits have been proposed. Automated view planning along the LP has shown to improve the quality of implantation, may speed up workflow and may reduce the need for low-dose aortograms. Rotational C-arm CT (RCT)-based view planning has proven to be of equal quality as MSCT-based techniques.
Current studies purely rely on non-quantitative evaluations and a systematic validation of software-based methods is lacking. RCT has proven to be a useful imaging technique for many clinical applications but is less established in the context of TAVR. According to the achieved clinical experience a more widespread use of RCT-based imaging could play a more significant role in the TAVR workflow in combination with software-based view planning support in the future.
This study is the first which combines the evaluation concerning the accuracy of a RCT- based 3D heart model for segmentation of the aortic root and prediction of the LP and its suitability for intervention guidance. The authors could show that their novel prototype software estimates optimal views on the basis of RCT data and that the model-based view planning in RCT has proven to be robust.
According to the authors’ results, RCT can be smoothly integrated into the clinical workflow, providing three-dimensional information of the relevant anatomical structures in the catheterization lab prior and as part of the TAVR intervention.
RCT is an imaging diagnostic tool to predict an optimal C-arm configuration during TAVR. RCT was performed as part of the pre-implant diagnostic coronary angiography study. To mitigate motion the acquisition was conducted during inspiratory breath hold and under rapid ventricular pacing. With prototype software each RCT data set was segmented using a 3D heart model. From that the LP curve was obtained that generates a perpendicular view of the aortic annulus according to the right-cusp rule. To evaluate the accuracy of a model-based overlay we compared model- and expert-derived aortic root diameters.
The authors are congratulated with their meticulous work on the use of rotational C-arm 3D heart model for prediction of an optimal C-arm configuration to be used before and during the procedure of transcatheter aortic valve replacement.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Farand P, Said SAM, Tagarakis G S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006-3008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2220] [Cited by in F6Publishing: 2094] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 2. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2682] [Cited by in F6Publishing: 2608] [Article Influence: 217.3] [Reference Citation Analysis (0)] |
| 3. | Tuzcu EM. Transcatheter aortic valve replacement malposition and embolization: innovation brings solutions also new challenges. Catheter Cardiovasc Interv. 2008;72:579-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Gurvitch R, Wood DA, Leipsic J, Tay E, Johnson M, Ye J, Nietlispach F, Wijesinghe N, Cheung A, Webb JG. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1157-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Samim M, Stella PR, Agostoni P, Kluin J, Ramjankhan F, Budde RP, Sieswerda G, Algeri E, van Belle C, Elkalioubie A. Automated 3D analysis of pre-procedural MDCT to predict annulus plane angulation and C-arm positioning: benefit on procedural outcome in patients referred for TAVR. JACC Cardiovasc Imaging. 2013;6:238-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Delgado V, Ng AC, Shanks M, van der Kley F, Schuijf JD, van de Veire NR, Kroft L, de Roos A, Schalij MJ, Bax JJ. Transcatheter aortic valve implantation: role of multimodality cardiac imaging. Expert Rev Cardiovasc Ther. 2010;8:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2012;6:366-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 472] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 8. | Tzikas A, Schultz C, Van Mieghem NM, de Jaegere PP, Serruys PW. Optimal projection estimation for transcatheter aortic valve implantation based on contrast-aortography: validation of a Prototype Software. Catheter Cardiovasc Interv. 2010;76:602-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Dvir D, Kornowski R. Percutaneous aortic valve implantation using novel imaging guidance. Catheter Cardiovasc Interv. 2010;76:450-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Poon KK, Crowhurst J, James C, Campbell D, Roper D, Chan J, Incani A, Clarke A, Tesar P, Aroney C. Impact of optimising fluoroscopic implant angles on paravalvular regurgitation in transcatheter aortic valve replacements - utility of three-dimensional rotational angiography. EuroIntervention. 2012;8:538-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Binder RK, Leipsic J, Wood D, Moore T, Toggweiler S, Willson A, Gurvitch R, Freeman M, Webb JG. Prediction of optimal deployment projection for transcatheter aortic valve replacement: angiographic 3-dimensional reconstruction of the aortic root versus multidetector computed tomography. Circ Cardiovasc Interv. 2012;5:247-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Krishnaswamy A, Tuzcu EM, Kapadia SR. Integration of MDCT and fluoroscopy using C-arm computed tomography to guide structural cardiac interventions in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2015;85:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Balzer JC, Boering YC, Mollus S, Schmidt M, Hellhammer K, Kroepil P, Westenfeld R, Zeus T, Antoch G, Linke A. Left ventricular contrast injection with rotational C-arm CT improves accuracy of aortic annulus measurement during cardiac catheterisation. EuroIntervention. 2014;10:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kasel AM, Cassese S, Leber AW, von Scheidt W, Kastrati A. Fluoroscopy-guided aortic root imaging for TAVR: “follow the right cusp” rule. JACC Cardiovasc Imaging. 2013;6:274-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Ecabert O, Peters J, Walker MJ, Ivanc T, Lorenz C, von Berg J, Lessick J, Vembar M, Weese J. Segmentation of the heart and great vessels in CT images using a model-based adaptation framework. Med Image Anal. 2011;15:863-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Manzke R, Meyer C, Ecabert O, Peters J, Noordhoek NJ, Thiagalingam A, Reddy VY, Chan RC, Weese J. Automatic segmentation of rotational x-ray images for anatomic intra-procedural surface generation in atrial fibrillation ablation procedures. IEEE Trans Med Imaging. 2010;29:260-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Waechter I, Kneser R, Korosoglou G, Peters J, Bakker NH, van der Boomen R, Weese J. Patient specific models for planning and guidance of minimally invasive aortic valve implantation. Med Image Comput Comput Assist Interv. 2010;13:526-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Korosoglou G, Gitsioudis G, Waechter-Stehle I, Weese J, Krumsdorf U, Chorianopoulos E, Hosch W, Kauczor HU, Katus HA, Bekeredjian R. Objective quantification of aortic valvular structures by cardiac computed tomography angiography in patients considered for transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2013;81:148-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Wollschläger H, Lee P, Zeiher A, Solzbach U, Bonzel T, Just H. Mathematical tools for spatial computations with biplane isocentric X-ray equipment. Biomed Tech (Berl). 1986;31:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Metropolis N, Ulam S. The Monte Carlo method. J Am Stat Assoc. 1949;44:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2416] [Cited by in F6Publishing: 945] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 21. | Schwartz JG, Neubauer AM, Fagan TE, Noordhoek NJ, Grass M, Carroll JD. Potential role of three-dimensional rotational angiography and C-arm CT for valvular repair and implantation. Int J Cardiovasc Imaging. 2011;27:1205-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |